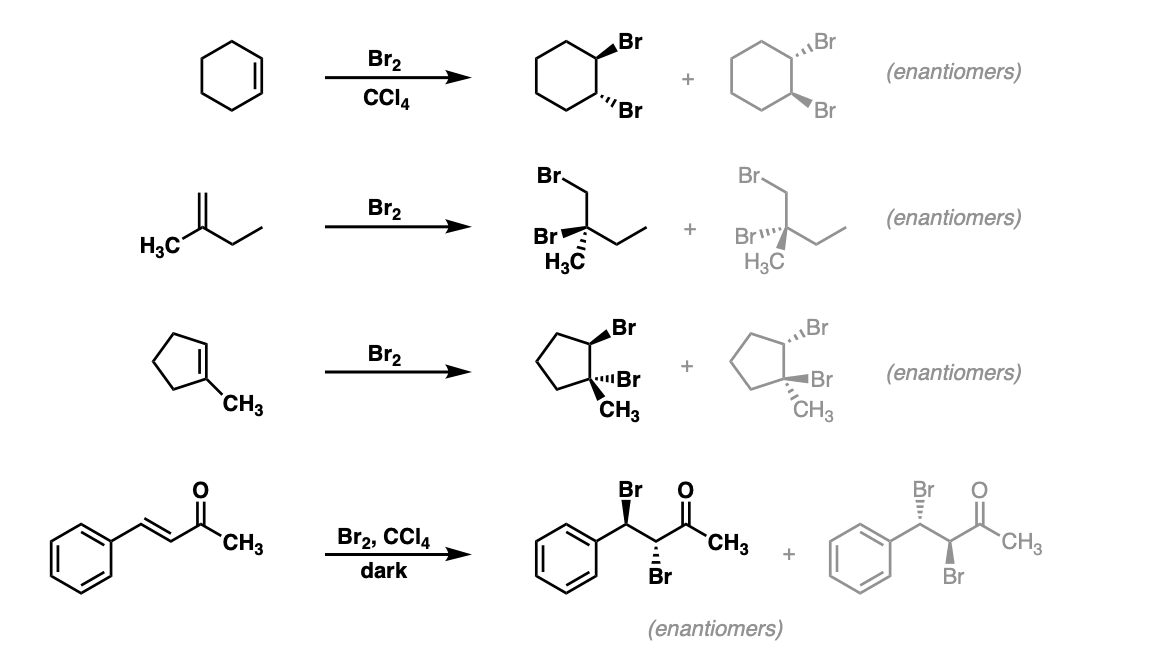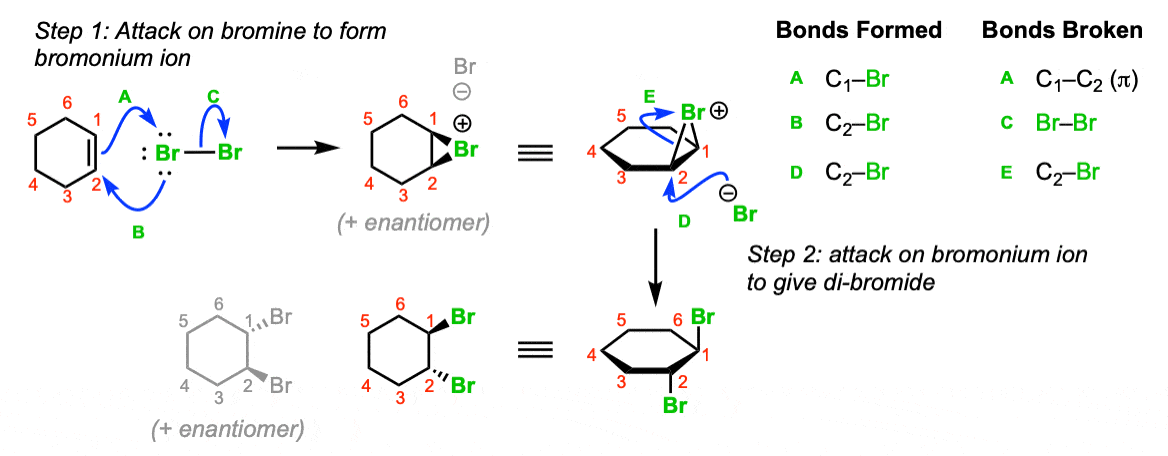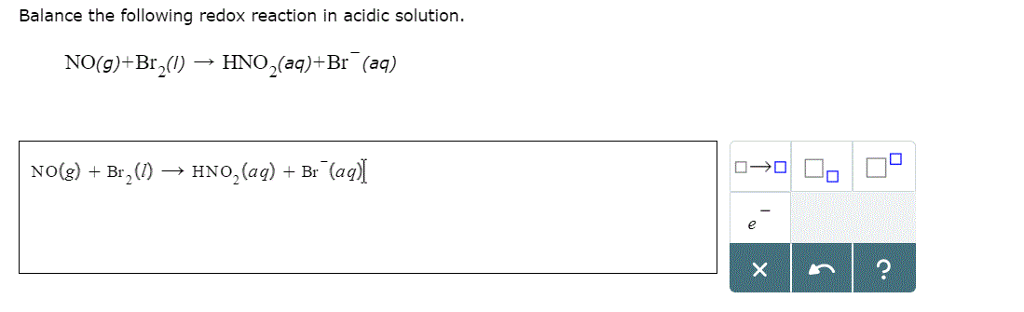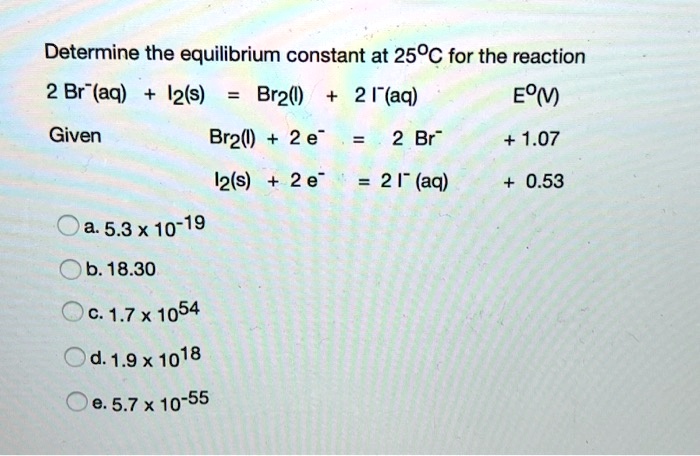
SOLVED: Determine the equilibrium constant at 250C for the reaction 2 Br" (aq) I2(s) Br2() 2 I(aq) EOm) Given Br2() 2 e" 2 Br" +1.07 I2(s) a. 5.3 X 10-19 b. 18.30
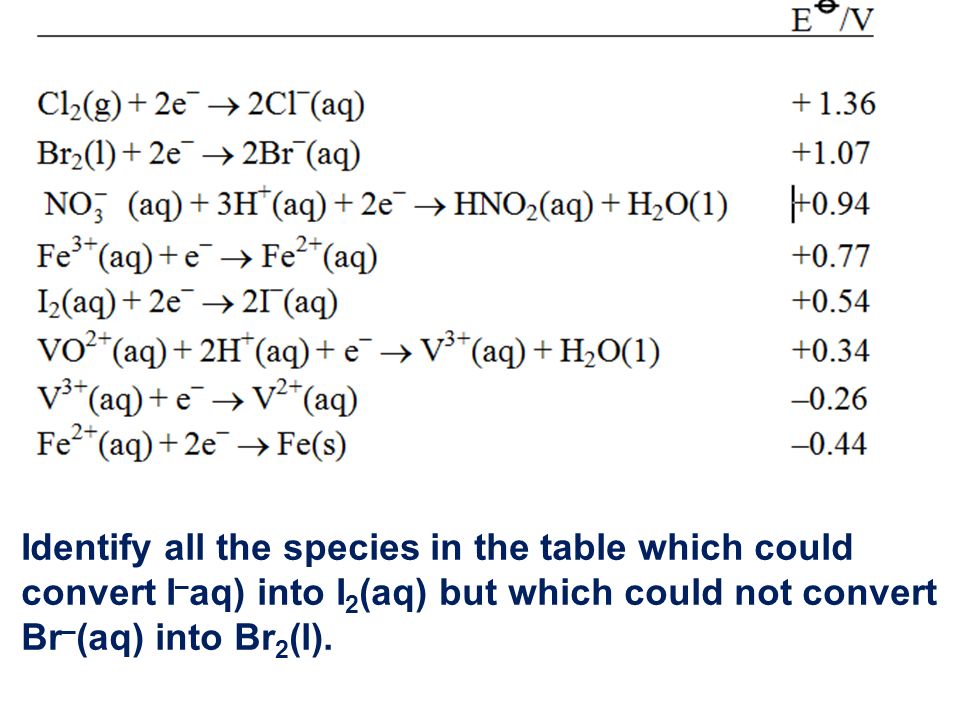
Q5 from 5.3 Identify all the species in the table which could convert I–aq) into I2(aq) but which could not convert Br–(aq) into Br2(l). - ppt video online download

SOLVED: Write a BALANCED equation for 4-methylcyclohexene with potassium permanaganate and a BALANCED equation for 4-methylcyclohexene with Bromine ( Br2). I am having trouble balancing the equation
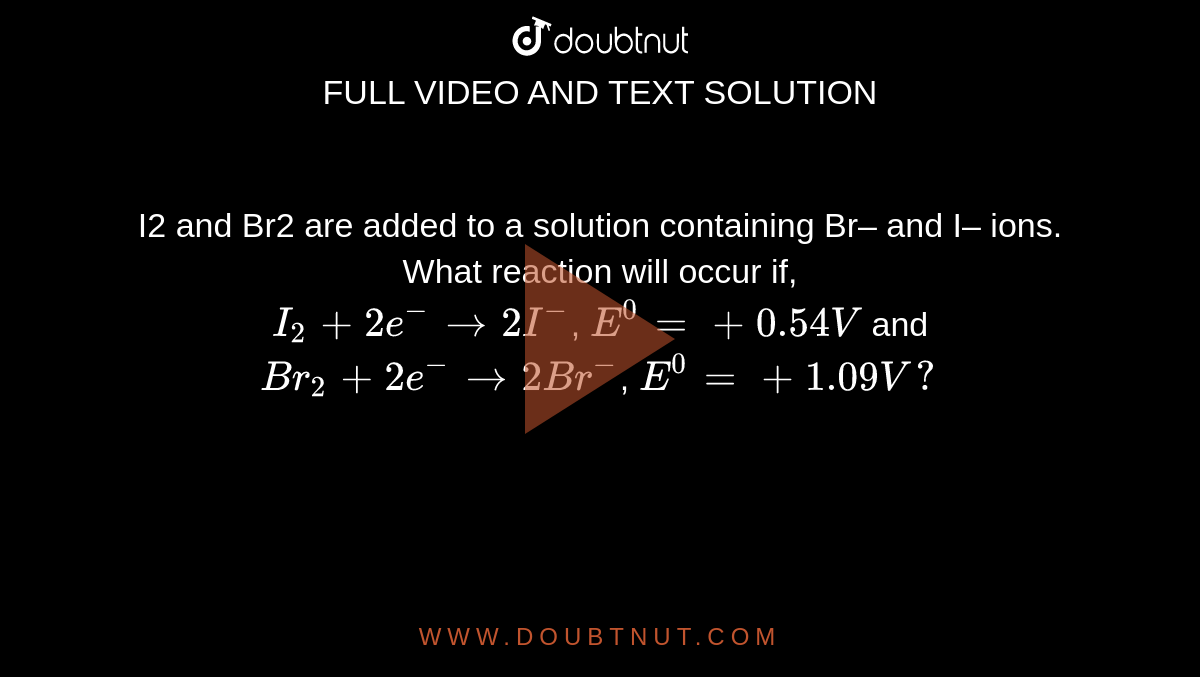
I2 and Br2 are added to a solution containing Br– and I– ions. What reaction will occur if, I(2) + 2e^(-) rarr 2I^(-), E^(0) = + 0.54V and Br(2) + 2e^(-) rarr 2Br^(-), E^(0) = +1.09 V?

SOLVED: determine the substance reduced this redox reaction 2 NaBr (s) + Cl2 (g) —> Br2 (I) + 2 NaCI (s) NaBr, Na, Cl2, NaCl, Br
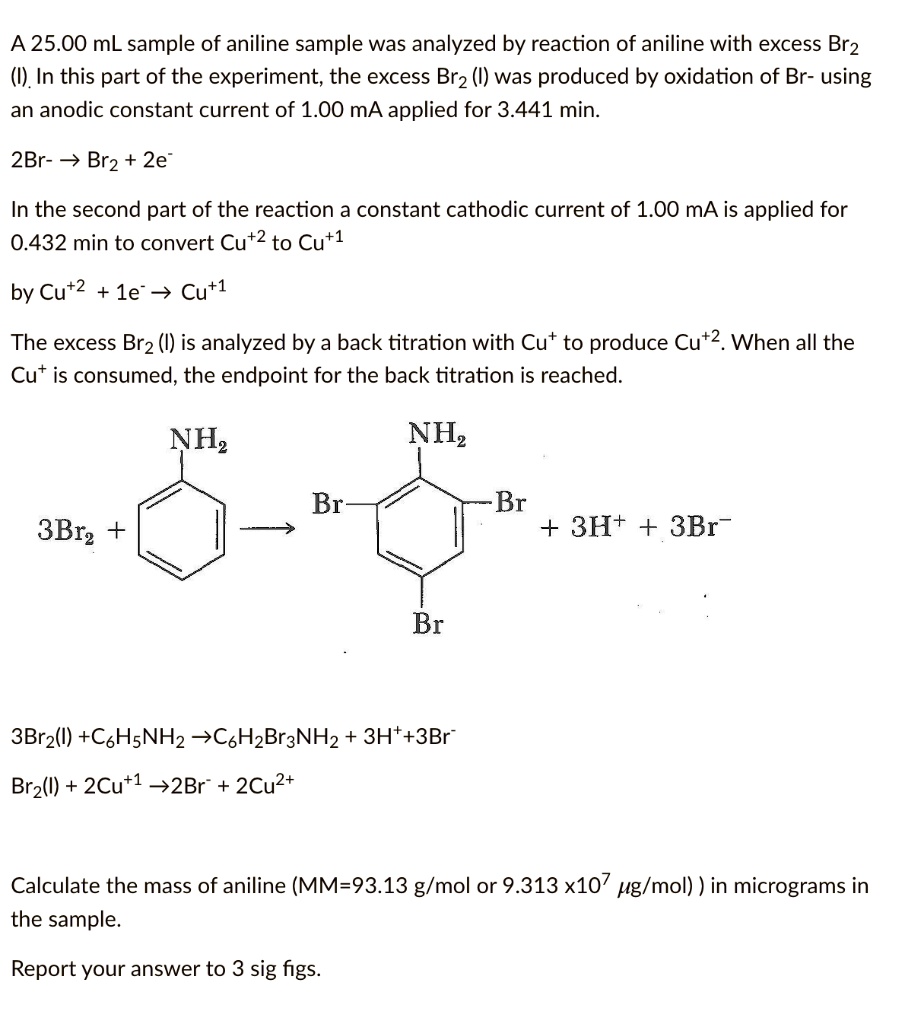




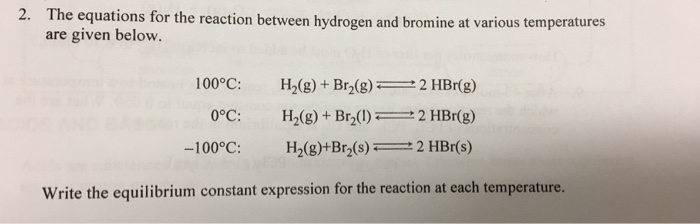

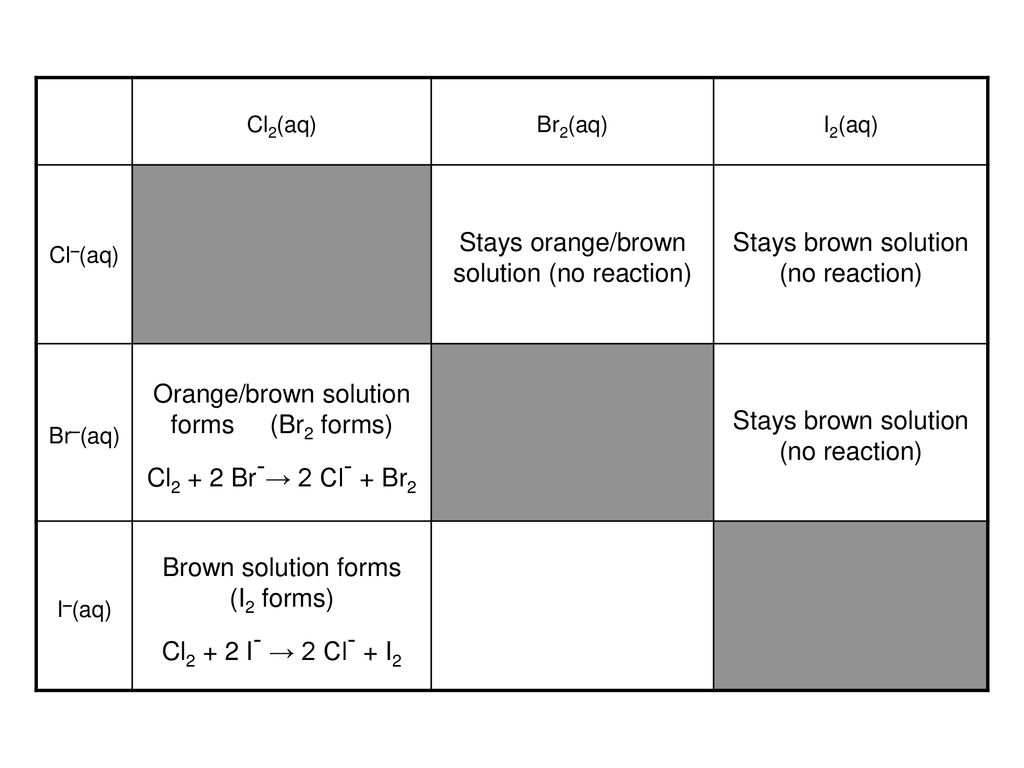
![Bromohydrin formation [Br2/H2O] - ChemistryScore Bromohydrin formation [Br2/H2O] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Bromohydrin-formation1-768x312.png)